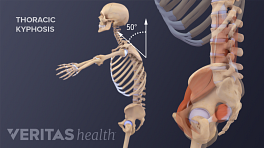
While one or more vertebral compression fractures can potentially cause back pain and an abnormal hunching forward (kyphosis), symptoms can typically be managed without surgery. If nonsurgical treatments fail to relieve the pain, a minimally invasive surgery called kyphoplasty may be considered.
In This Article:
- Kyphoplasty for Vertebral Compression Fractures
- Kyphoplasty Procedure Overview
- Kyphoplasty Risks and Potential Complications
- Kyphoplasty (Osteoporosis Fracture Treatment) Video
How Kyphoplasty Can Treat a Compression Fracture
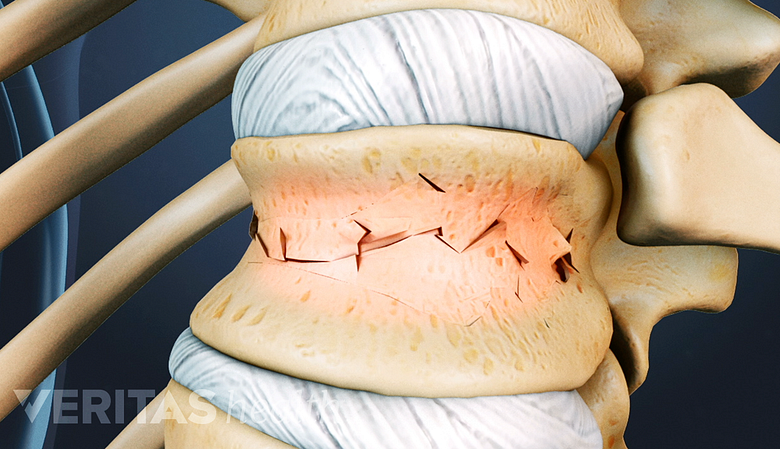
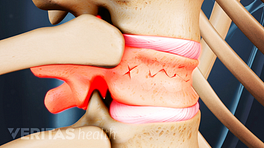
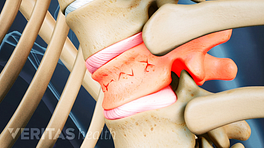
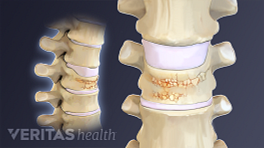
The vertebral body is the strong, thick cylindrical front of the vertebral bone that plays a crucial role in handling large axial loads placed on the spine. When a vertebral body becomes weakened, such as from osteoporosis, it is less capable of handling axial loads and more susceptible to vertebral compression fractures. The most common type of vertebral fracture is a wedge fracture, which involves the front part of the vertebral body collapsing at least 15% compared to its normal height.
See Vertebral Fracture Symptoms
A kyphoplasty procedure uses x-ray guidance to place a needle through a small incision in the back and into the vertebral compression fracture. After the needle is accurately placed, a balloon is slowly inflated to help restore vertebral height and form a new cavity. Bone cement is then injected into the new cavity and quickly hardens, which alleviates pain by strengthening and solidifying the damaged vertebra.
When to Consider Kyphoplasty
Kyphoplasty is considered for progressive back pain symptoms caused by a compression fracture.
Most vertebral compression fractures heal on their own as the pain eventually goes away. In cases where a vertebral compression fracture has been verified via x-ray and significant pain persists for more than a couple weeks despite nonsurgical treatments, kyphoplasty may be considered if the following are true:
- Pain worsens when weight is applied. If back pain worsens when axial loads are applied to the spine, that is an indication that the vertebral compression fracture is indeed the pain source and could be treated with a kyphoplasty or other vertebral augmentation procedure. Clues that axial load worsens pain include increased pain when carrying heavy items, such as groceries or suitcases, or getting into and out of bed.
- Pain is not accompanied by tingling, numbness, or weakness. Kyphoplasty is unlikely to relieve neurological symptoms of tingling, numbness, or weakness that occur when part of the vertebra pushes against the spinal cord or nerve root. If the vertebra has started to cause symptomatic nerve inflammation, fusion surgery may be recommended instead.
- Vertebra collapses between 30% and 70%. Due to its ability to restore some vertebral height, kyphoplasty is typically not recommended unless the front part of the vertebral body has collapsed at least 30% compared to the back of the vertebral body. In cases where the vertebral body has collapsed less than 30%, the simpler vertebroplasty procedure might be recommended because stabilizing the fracture without restoring vertebral height may be sufficient. Furthermore, kyphoplasty is not recommended if the vertebral body has collapsed more than 70%, in which case a more extensive surgery may be needed.
- Fracture is less than 3 months old. Vertebral compression fractures that occurred more than 3 months prior to surgery have already started to heal and are unlikely to experience the vertebral height restoration normally associated with kyphoplasty.
Other factors to consider before deciding on kyphoplasty also exist, such as whether the patient is healthy enough for surgery. For example, if the patient is of advanced age or has the compression fracture as a result of a bone infection, the surgery is unlikely to be well tolerated.
Efficacy of Kyphoplasty
Current medical literature indicates that kyphoplasty is an effective treatment for restoring vertebral height and eliminating pain from vertebral compression fractures. Compared to a similar procedure called vertebroplasty, which stabilizes the fracture without restoring vertebral height, kyphoplasty appears to offer the same amount of pain relief with similar risks. 1 Sebaaly A, Nabhane L, El Khoury FI, Kreichati G, El Rachkidi R. Vertebral augmentation: state of the art. Asian Spine J. 2016; 10(2): 370-6. , 2 Stevenson M, Gomersall T, M Lloyd Jones et al. Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral fractures: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014; 18(17):1-290.
Some studies have also found that kyphoplasty (and vertebroplasty) may help older adults who have had a vertebral compression fracture to live longer. 3 Chen AT, Cohen DB, Skolasky RL. Impact of nonoperative treatment, vertebroplasty, and kyphoplasty on survival and morbidity after vertebral compression fracture in the medicare population. J Bone Joint Surg Am. 2013; 95(19):1729-36. , 4 Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res. 2011; 26(7):1617-26. One possible reason may be that, compared to nonsurgical treatments for vertebral compression fractures, kyphoplasty may help the patient achieve better functioning to protect against future falls or other serious complications. However, more research is needed.
- 1 Sebaaly A, Nabhane L, El Khoury FI, Kreichati G, El Rachkidi R. Vertebral augmentation: state of the art. Asian Spine J. 2016; 10(2): 370-6.
- 2 Stevenson M, Gomersall T, M Lloyd Jones et al. Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral fractures: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014; 18(17):1-290.
- 3 Chen AT, Cohen DB, Skolasky RL. Impact of nonoperative treatment, vertebroplasty, and kyphoplasty on survival and morbidity after vertebral compression fracture in the medicare population. J Bone Joint Surg Am. 2013; 95(19):1729-36.
- 4 Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res. 2011; 26(7):1617-26.