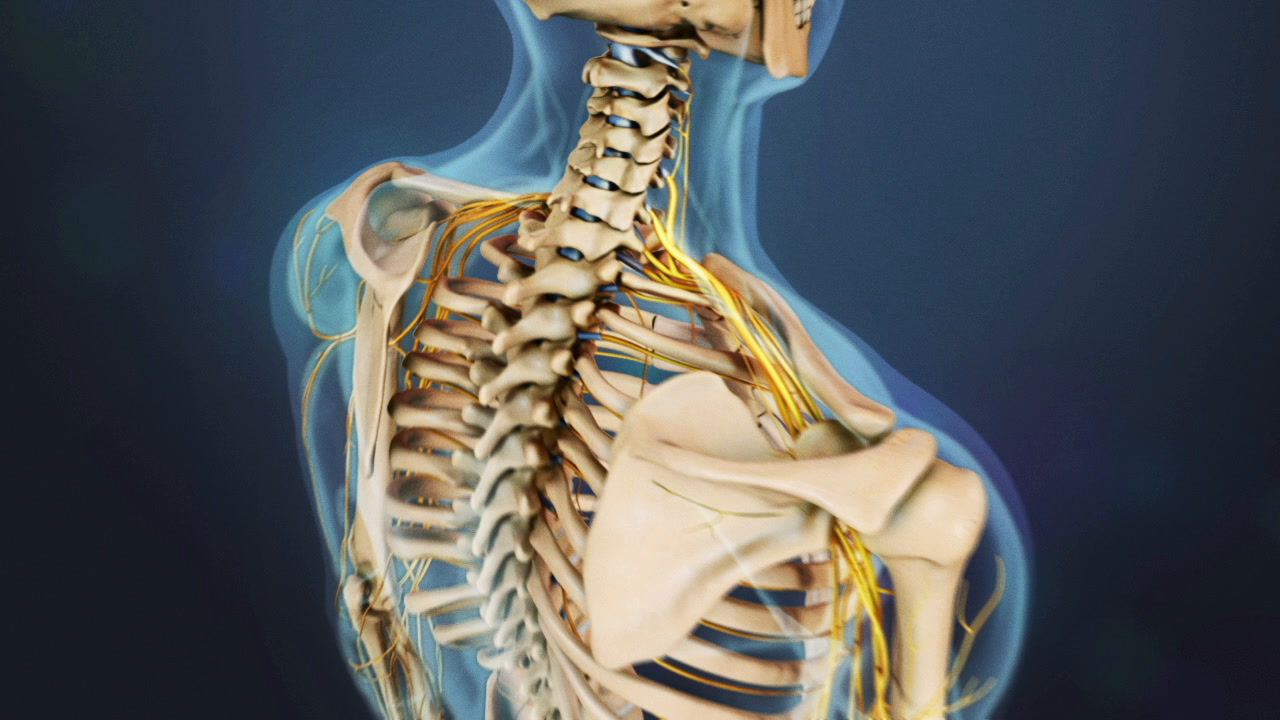
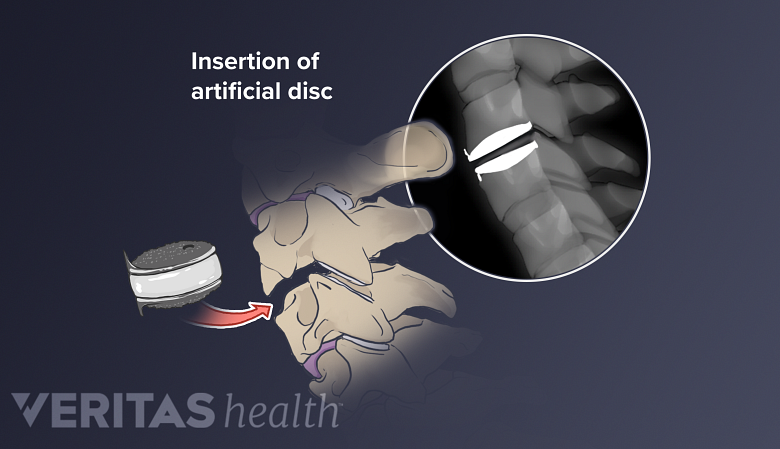
Several cervical artificial disc technologies have been developed to replace degenerated intervertebral discs in the cervical spine. While no artificial disc can perfectly replace a natural disc’s ability to cushion and transfer loads in the neck, an artificial disc may maintain more of the cervical spine’s natural range of motion compared to fusion surgery.
When a cervical artificial disc replacement surgery is performed, choosing an artificial disc requires careful mechanical and biological considerations.
In This Article:
- Cervical Artificial Disc Replacement Technologies
- Cervical Artificial Discs: Fusion, Stability and Wear
- Cervical Disc Replacement Surgery Video
Goals of Artificial Cervical Discs
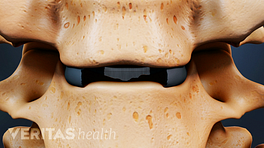
Artificial cervical discs restore the correct spacing between vertebrae.
An ideal artificial disc will usually achieve the following goals:
- Cover endplates. Endplates contain cartilage that separates the disc from the vertebral bone both above and below. When an artificial disc is large enough to fully cover these endplates, it results in better distribution of loads on the cervical spine. 1 Thaler M, Hartmann S, Gstöttner M, Lechner R, Gabl M, Bach C. Footprint mismatch in total cervical disc arthroplasty. European Spine Journal. 2012;22(4):759-765. doi:10.1007/s00586-012-2594-3
- Relieve nerve compression with the shortest height possible. The height of the artificial disc plays an important role in preventing nerve root compression. Discs with the smallest height that can achieve complete nerve decompression are better than taller discs. Taller discs may cause the facet joints and other connective tissues joining the vertebrae to pull away from each other, resulting in decreased motion. 2 Li H, Lou J, Liu H, Wang B. Effect of intervertebral disc height on the range of motion and clinical outcomes after single-level implantation of Prestige LP cervical disc prosthesis. Clinical Neurology and Neurosurgery. 2016;148:1-4. doi:10.1016/j.clineuro.2016.06.010
Artificial discs are available in various sizes, shapes, and heights in order to achieve these goals and provide good surgical outcomes. Several types of discs have been fabricated using different materials, designs, and techniques.
Materials Used to Create Artificial Cervical Discs
The materials used to make artificial discs must be strong, corrosion-resistant, and able to bear loads from the head’s weight and during neck movements.
Common materials used to create artificial cervical discs include:
- Polyethylene, a nonmetallic polymer made up of long molecular chains that can effectively transfer loads and provide high impact strength to the artificial disc.
- Titanium, a strong and corrosion-resistant metal with good elastic properties that encourages the growth of bone on the disc, an important feature for long-term stability. 3 Pham MH, Mehta VA, Tuchman A, Hsieh PC. Material Science in Cervical Total Disc Replacement. BioMed Research International. 2015;2015:1-9. doi:10.1155/2015/719123
- Cobalt-chrome, a mixture of cobalt and chromium metals, forming an alloy of high strength and stiffness, while also being corrosion resistant.
- Stainless steel, made by mixing iron and carbon produces strong discs, although its mechanical properties may be inferior to its metallic counterparts.
Since most artificial discs approved for use in the US have only been available for about a decade or less, more time is needed to study the long-term effects of these materials on the body.
See Potential Complications and Risks of Cervical Disc Replacement Surgery
Medical imaging difficulties with artificial discs
The metallic components of an artificial disc may produce artifacts (abnormality seen on imaging tests but does not actually exist) on magnetic resonance imaging (MRI) scans. These artifacts may lead to diagnostic difficulties postoperatively and in the future. Polyethylene, however, does not create such artifacts.
Design Concepts of Artificial Cervical Discs
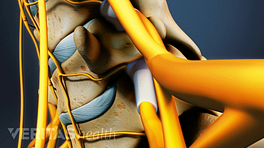
The artificial disc replicates both the structure and function of the natural disc.
A disc typically has two or more parts to allow movement and articulation.
Bearing types
Artificial discs are available in various types. The parts of an artificial disc may be constrained (fused), semi-constrained (partially fused), or unconstrained (independent). The constrained discs usually have less mobility but good stability, while unconstrained discs have more mobility but decreased stability. Semi-constrained discs have moderate stability and mobility.
Articulation
The parts of an artificial cervical disc are mechanically connected using one of the following 2 articulating methods:
- Ball-and-socket. A ball-and-socket type of disc allows rotation of the spinal motion segment around one fixed point. This design has the following features:
- Typically, there are two metallic plates with a metallic or polyethylene ball in between. The ball is the primary point of rotation.
- The ball may be an independent piece or may be attached to the inner surface of the lower endplate.
- The shape of the ball may vary in different types of discs, some featuring a piece of polyethylene with curved upper and lower surfaces.
- Some designs may have a slight concavity in the inner surface of the upper endplate for the ball to lodge itself.
- Saddle. A saddle type of disc is made of 2 pieces and allows movement of the spinal motion segment at more than one point. This design has the following features:
- A center of rotation fused on the lower piece allows forward and backward movements.
- A center of rotation fused on the upper piece allows side-to-side bending.
At the back, the discs have stops or elongated parts of metal that prevent them from slipping or being dislocated into the spinal canal.
Various techniques have been developed to implant an artificial disc into a spinal motion segment. While artificial cervical discs may maintain more of the spine’s natural mobility compared to fusion surgery, they must be durable enough to handle daily wear over time.
See Artificial Disc Replacement or Spinal Fusion: Which is Better for You?
- 1 Thaler M, Hartmann S, Gstöttner M, Lechner R, Gabl M, Bach C. Footprint mismatch in total cervical disc arthroplasty. European Spine Journal. 2012;22(4):759-765. doi:10.1007/s00586-012-2594-3
- 2 Li H, Lou J, Liu H, Wang B. Effect of intervertebral disc height on the range of motion and clinical outcomes after single-level implantation of Prestige LP cervical disc prosthesis. Clinical Neurology and Neurosurgery. 2016;148:1-4. doi:10.1016/j.clineuro.2016.06.010
- 3 Pham MH, Mehta VA, Tuchman A, Hsieh PC. Material Science in Cervical Total Disc Replacement. BioMed Research International. 2015;2015:1-9. doi:10.1155/2015/719123