Discover the power of personalized pain relief
Because your pain is profoundly personal, you are more likely to find lasting relief with a solution that can be personalized to your unique pain.
Boston Scientific’s pain management solutions have helped thousands of people find relief from pain—even when other therapies have failed.
Treating chronic pain with Spinal Cord Stimulation (SCS)
You feel pain when nerves send pain signals through your spinal cord to your brain. SCS is designed to interrupt these signals so you don’t perceive pain.
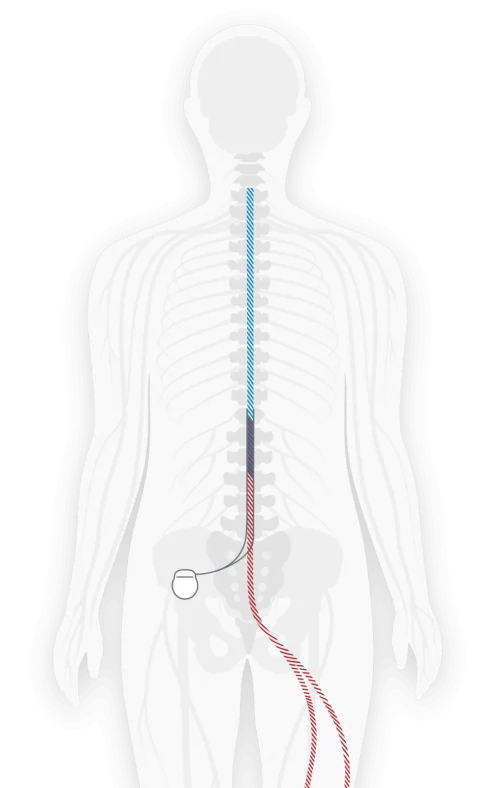
How SCS works

Stimulator A small device called a spinal cord stimulator is implanted under the skin.

Leads Thin, flexible “leads” are connected to the stimulator and placed near specific nerves along the spinal cord.

Pulses The stimulator sends mild pulses through the leads to the nerves.

Pain The pulses interrupt the pain signal on the way to the brain.
SCS from Boston Scientific:
Multiple therapies. One device.
There are different kinds of SCS therapies.
Some therapies replace your pain with a tingling sensation. Others use a type of stimulation you don't feel at all.
With Boston Scientific, you can choose the therapy that works best for you—or use both at the same time.
The ability to deliver multiple therapies simultaneously* is unique to Boston Scientific SCS Systems and has been clinically proven to provide greater pain relief.1
In a clinical study, patients using Boston Scientific's SCS therapies reported a greater improvement in their ability to do everyday activities† than patients in other studies using non-Boston Scientific SCS Systems.2,3,4
Cognita™ Care: Learn, Connect, and Thrive
At Boston Scientific, we're committed to helping you manage chronic pain so you can get back to living life to the fullest. That's why we created Cognita Care, with personalized support and innovative tools to help you find effective, long-term pain relief.
Personalized support. Long-term relief.
Finding long-term relief from chronic pain is a journey that begins with learning about different solutions that might be right for you, and then connecting with specialists who can help. Cognita Care helps make that journey easier, with cutting-edge digital tools and in-person services to help you find effective long-term solutions for your personal pain.

Learn
Learn about your therapy options

Connect
Connect with specialists who can help you find lasting pain relief

Thrive
Access resources so you can continue to thrive
*Paresthesia-free stimulation has been shown to be safe and effective in patients who have been treated successfully with paresthesia-inducing stimulation for at least 6 months.
- North, James, MD. WHISPER: A Multicenter, Prospective Randomized Controlled Trial Evaluating Subperception SCS at ≤ 1.2 kHz. Presentation at North American Neuromodulation Society (NANS), Las Vegas, NV, January 11-14, 2018. (N=70)
- Wallace M. et al. Outcomes of a Prospective Randomized Controlled Trial Utilizing a Spinal Cord System Capable of Multiple Neurostimulation Modalities (COMBO). NANS Annual Meeting. January 2020 (N=89)
- Kapural, Cong Yu, et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology 2015; 123:851 -860
- Deer, Timothy, et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation: Technology at the Neural Interface. 21. 10.1111/ner.12698
Results from clinical studies are not predictive of results in other studies. Results in other studies may vary.
US Indications for Use: The Boston Scientific Spinal Cord Stimulator (SCS) Systems* are indicated as an aid in the management of chronic intractable pain of the trunk and/or limbs including unilateral or bilateral pain associated with the following: Failed Back Surgery Syndrome, Complex Regional Pain Syndrome (CRPS) Types I and II, Intractable low back pain and leg pain, Diabetic Peripheral Neuropathy of the lower extremities, Radicular pain syndrome, Radiculopathies resulting in pain secondary to failed back syndrome or herniated disc, Epidural fibrosis, Degenerative disc disease (herniated disc pain refractory to conservative and surgical interventions), Arachnoiditis, Multiple back surgeries.
The Boston Scientific Spectra WaveWriter™, WaveWriter Alpha™ and WaveWriter Alpha™ Prime SCS Systems are also indicated as an aid in the management of chronic intractable unilateral or bilateral low back and leg pain without prior back surgery.
*The Boston Scientific Spinal Cord Stimulator (SCS) Systems include the following: Precision™ System, Precision Spectra™ System, Precision Novi™ System, Precision Montage™ MRI System, Spectra WaveWriter™ System, WaveWriter Alpha™ System, WaveWriter Alpha™ Prime System
Note: CRPS I was previously referred to as Reflex Sympathetic Dystrophy (RSD) and CRPS II was previously referred to as causalgia.
The mySCS™ Go Therapy Controller is intended to communicate with and control the compatible Boston Scientific Stimulator.
Contraindications: The Boston Scientific Spinal Cord Stimulator systems are not for patients who are unable to operate the system, have failed trial stimulation by failing to receive effective pain relief, are poor surgical candidates, or are pregnant.
Warnings: With all medical procedures, there are risks associated with the procedure and the use of the device. Patients implanted with Boston Scientific Spinal Cord Stimulator systems without ImageReady™ MRI Technology should not be exposed to Magnetic Resonance Imaging (MRI). Exposure to MRI may result in dislodgement of the stimulator or leads, heating of the stimulator, severe damage to the stimulator electronics and an uncomfortable or jolting sensation. As a Spinal Cord Stimulation patient, you should not have diathermy as either a treatment for a medical condition or as part of a surgical procedure. Strong electromagnetic fields, such as power generators or theft detection systems, can potentially turn the stimulator off, or cause uncomfortable jolting stimulation. The system should not be charged while sleeping. The Boston Scientific Spinal Cord Stimulator system may interfere with the operation of implanted sensing stimulators such as pacemakers or implanted cardiac defibrillators. Advise your physician that you have a Spinal Cord Stimulator before going through with other implantable device therapies so that medical decisions can be made and appropriate safety measures taken. Patients using therapy that generates paresthesia should not operate motorized vehicles such as automobiles or potentially dangerous machinery and equipment with the stimulation on. Stimulation must be turned off first in such cases. For therapy that does not generate paresthesia (i.e. subperception therapy) it is less likely that sudden stimulation changes resulting in distraction could occur while having stimulation on when operating moving vehicles, machinery, and equipment.
Be sure to talk with your doctor so that you thoroughly understand all of the risks, precautions, and benefits associated with the use of the device and what indicates, and contraindicates, certain patients– as well as the risks and precautions for the procedure. For complete indications for use, contraindications, warnings, precautions, and side effects, call 866.360.4747 or visit Pain.com.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Copyright © 2025 by Boston Scientific Corporation or its affiliates. All rights reserved.
NM-1017712-AC