Water therapy or aquatic therapy is an excellent choice for anyone who has too much back pain and/or joint pain for land-based physical therapy.
McKenzie therapy is not advised for treating pain from serious back or neck injuries, after spine surgery, or in cases where the pain does not centralize.
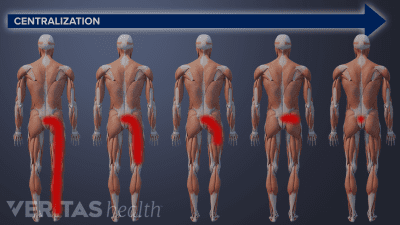
You may experience significant pain relief after just one session of McKenzie exercises, but it takes at least 1 to 3 weeks of therapy to get sustained pain relief.
How to relieve SI joint pain while sitting or sleeping? Learn essential tips on ergonomic adjustments, posture, and sleeping position changes to help reduce SI joint pain while sitting or sleeping.
Learn about the differences between sacroiliitis and arthritis. It’s important to note that sacroiliitis is linked to inflammatory arthritis but can occur independently.
Discover the connection between your diet and SI joint pain, and how making dietary changes can help reduce inflammation and alleviate pain.
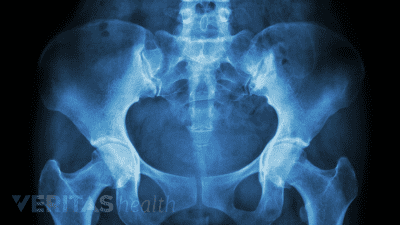
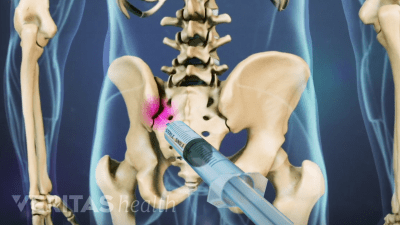
Discover the best radiographic tests for diagnosing SI joint dysfunction and ruling out serious medical conditions.
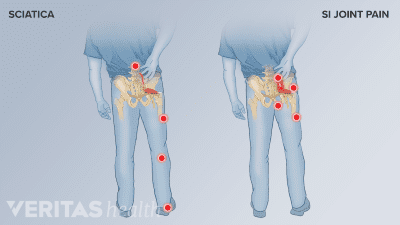
Sacroiliitis and sciatica may have similar symptoms, but their causes and treatments are different. Find out which one is causing your pain and how to treat it.
SI joint pain can last days to months and worsen if left untreated. Learn how to relieve SI joint pain quickly.
Discover how yoga can help relieve SI joint pain by increasing flexibility and improving posture. Follow these simple poses to strengthen your muscles.