Featured Health Topics
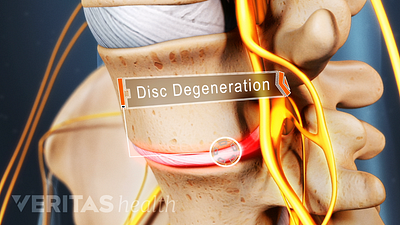
What Is Degenerative Disc Disease?
Contrary to the name, degenerative disc disease doesn't necessarily worsen with age, but it can lead to severe pain.
Neck Stretches
Neck stretching exercises can help loosen postural muscles and may reduce neck pain.
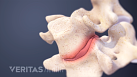
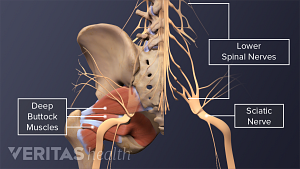
Sciatica Causes
The origins and risk factors of sciatica can be related to several underlying causes.
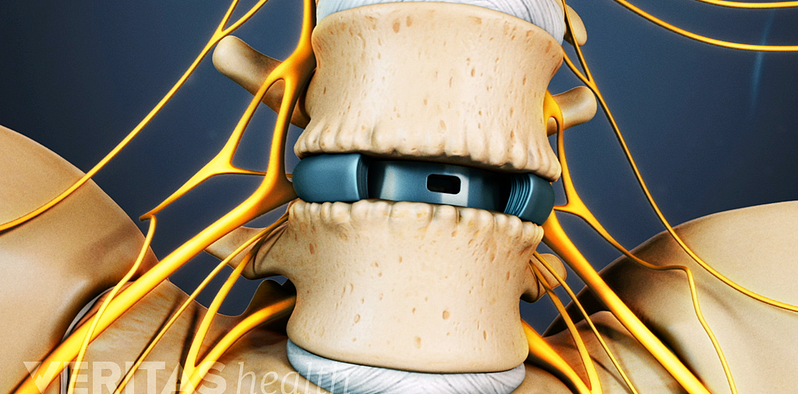
Lumbar Spine Surgery
Spotlight on your health
Lower Back Pain Causes and Symptoms Video
Chronic pain in the lower back may be caused by the discs or the joints of the spine.
Cervical Spinal Stenosis Video
Learn about cervical stenosis and its symptoms, causes and treatment options in this interactive cervical spinal stenosis video.
Sciatica Causes and Symptoms Video
Sciatica is leg pain caused by a problem in the low back. Watch an animated video that details the causes and symptoms of sciatica.
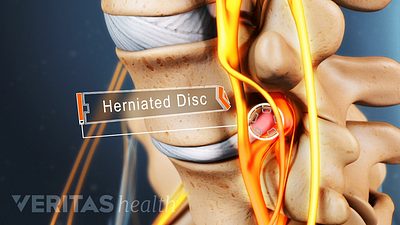
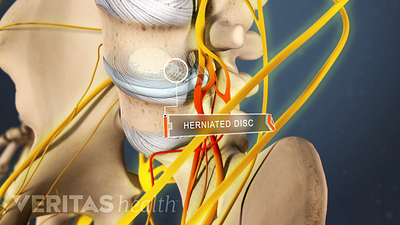
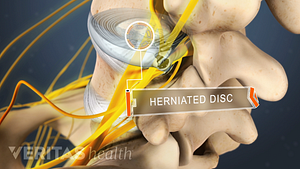
Lumbar Herniated Disc Video
A herniated disc in the lumbar spine can put pressure on spinal nerve roots, causing pain in the lower back or legs.
Editors Top Picks
Relieve sciatica pain and prevent future flare-ups by stretching and strengthening your lower back, abdominal, and thigh muscles with these 3 simple exercises.
A complete guide to the causes of buttock muscle pain, sciatica pain in the buttock, and treatments to help relieve pain in the buttock area.
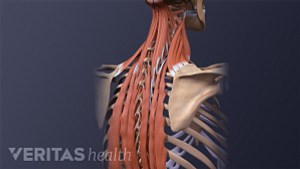
Pain in the shoulder blade region is fairly common and can be quite painful and limiting. It can have many causes, ranging from a muscle strain to a more serious condition that requires immediate medical attention.
Home remedies that treat inflammation and reduce pressure on the spinal discs provide pain relief and facilitate healing from a herniated disc in the lower back.