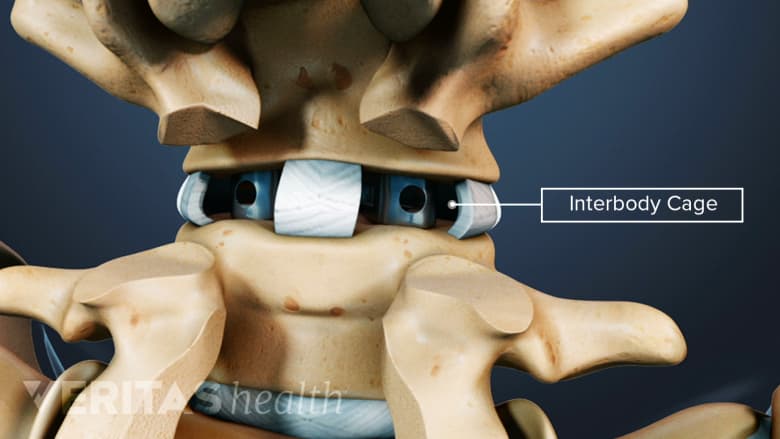
Interbody cages are cylindrical or crescent-shaped, hollow, and porous metallic devices that are placed between two adjacent vertebrae in a spinal segment after removing the damaged disc. The cage may occupy the entire disc space or just the front (anterior) part of it. A spinal surgery that warrants removal of the disc, such as interbody fusion, usually uses interbody cages.
The top and bottom of a spinal interbody cage are in contact with the vertebral endplates—the transition region where a vertebral body and intervertebral disc interface with each other. The cage is filled with bone graft material (harvested from the patient's body or obtained from another source) that grows over time and fills within the interbody space, permanently fusing the two adjacent vertebrae of the treated segment.
In This Article:
- Spine Fusion Instrumentation
- Pedicle Screws for Spine Fusion
- Interbody Cages for Spine Fusion
Interbody Cage Designs
The design of a cage typically depends on its material, placement, and technique used to deliver it into the interbody or disc space of the spine. A cage that covers more surface area of the vertebral endplates, such as a patient-specific 3D printed cage, helps achieve higher stability by decreasing the forces directly impacting the unsupported area(s) of the endplates.1Fernandes RJR, Gee A, Kanawati AJ, et al. Evaluation of the contact surface between vertebral endplate and 3D printed patient-specific cage vs commercial cage. Sci Rep. 2022;12(1):12505. Published 2022 Jul 22. doi:10.1038/s41598-022-16895-9
Anterior interbody cages
Anterior interbody cages are titanium cylinders that are placed in the disc space. These cages are porous and hollow, and allow the bone graft material within them to grow from one vertebral body to the other. The cages are threaded and attach well to the spine, so most patients do not need additional instrumentation, such as pedicle screws or post-surgical back braces for fixation and support.
A potential advantage of an anterior interbody cage is that it allows the placement of a larger implant, thereby decreasing the risk of a failed fusion (pseudoarthrosis) and providing better expansion of bony spaces for the spinal nerves.2Sasaki M, Kishima H. [Standard Techniques of Spinal Fusion for Lumbar Degenerative Diseases]. No Shinkei Geka. 2021;49(6):1257-1270. doi:10.11477/mf.1436204512 Cylindrical cages are usually used when the height of the disc space becomes compressed or collapsed due to disc degeneration or wearing. While the threads on the cage help to achieve better fusion rates than non-threaded cages, threads also decrease the stability of the cage. More recent designs are non-threaded cages that are box-shaped, intended to have greater stability and therefore lower risk of subsidence (change in height and shape over time).3Jain S, Eltorai AE, Ruttiman R, Daniels AH. Advances in Spinal Interbody Cages. Orthop Surg. 2016;8(3):278-284. doi:10.1111/os.12264
Tall disc-shaped metallic cages
Recent technology has developed metallic cages that can firmly grip the disc space and more closely match its anatomy. Because this cage is disc-shaped, it can be used in disc spaces that have retained the normal height of the disc.
Posterior interbody cages
Two rectangular cages placed in the evacuated disc space during a posterior lumbar interbody fusion (PLIF) surgery.
Posterior interbody cages are made of the same materials and designs as anterior interbody cages but are placed into the disc space from the back of the spine.
Expandable cages
Cages can be designed to expand after insertion, specifically after a surgery in which the vertebral body is partially or fully removed. The purpose of the expandable cages is to restore the shape of the spine with greater control and ease. Patients with osteoporosis or a history of radiation treatment for metastatic spinal tumors may have poor bone density and may benefit from the use of expanding cages. These cages reduce the risk of damage to the vertebral endplates.4
Drawbacks of expandable cages include a decreased surface area for spinal fusion, which may lead to incomplete fusion and possible revision surgery.5 Also, expandable cages are typically more expensive than non-expandable cages, making it challenging to get approvals for these devices from hospitals and/or payors.
See What Are Cages and Why Is Your Surgeon Putting One in Your Spine?
Interbody Cage Placement
Most of the cages are placed in the front of the spine through an anterior lumbar interbody fusion (ALIF) surgery. The cages can be inserted through a small incision (mini-laparotomy) or with an endoscope (a small tube with a camera at the end that allows the surgery to be done through several one-inch incisions).
By far, the most popular approach for inserting titanium cages is through a mini-laparotomy, as the endoscopic approach is difficult and does not provide good visibility of the spinal segment. These cages may also be placed in the back of the spine through a posterior lumbar interbody fusion (PLIF) surgery.
Success Rates of Spinal Cages
Cages that remain in place, maintain their shape and height, and allow the bone graft to fuse are considered stable and successful. The stability of a cage is determined by measuring its subsidence—the degree of sinkage or caving in. A successful cage design is resistant to subsidence.
- Threaded cylindrical cages are associated with a fusion rate of 93% to 96%, with good or excellent pain improvement in 65% to 72% of cases.6
- Cages made of PEEK (polyether ether ketone) typically have a higher rate of successful fusion, lower rate of subsidence, and a greater chance of restored disc height than cages made of titanium.7
The cage’s design, material, and height; types of instruments used; and the surgeon’s expertise in placing the hardware in the spine are additional factors that contribute to its success.
Potential Risks and Complications of Spinal Cages
The risks and possible complications of a spinal cage would include all the possible risks of spinal surgery. Rarely, spinal cages may be subject to breakage or dislodgement.
If cage subsidence occurs, it may contribute to progressive complications, such as segmental kyphosis, the development of degeneration in adjacent vertebral segments, and spinal stenosis (the narrowing of the foramina where nerve roots pass).8Jin ZY, Teng Y, Wang HZ, Yang HL, Lu YJ, Gan MF. Comparative Analysis of Cage Subsidence in Anterior Cervical Decompression and Fusion: Zero Profile Anchored Spacer (ROI-C) vs. Conventional Cage and Plate Construct. Front Surg. 2021;8:736680. Published 2021 Oct 27. doi:10.3389/fsurg.2021.736680
Spinal interbody cages are useful tools in fusion surgeries and serve as a space holder between the treated vertebral levels. Cages allow bone to grow through it, eventually becoming a part of the spine. Discussing the type and material of the cage with the surgeon can help patients understand the advantages and disadvantages of this implant in their spine.
- 1 Fernandes RJR, Gee A, Kanawati AJ, et al. Evaluation of the contact surface between vertebral endplate and 3D printed patient-specific cage vs commercial cage. Sci Rep. 2022;12(1):12505. Published 2022 Jul 22. doi:10.1038/s41598-022-16895-9
- 2 Sasaki M, Kishima H. [Standard Techniques of Spinal Fusion for Lumbar Degenerative Diseases]. No Shinkei Geka. 2021;49(6):1257-1270. doi:10.11477/mf.1436204512
- 3 Jain S, Eltorai AE, Ruttiman R, Daniels AH. Advances in Spinal Interbody Cages. Orthop Surg. 2016;8(3):278-284. doi:10.1111/os.12264
- 4
- 5
- 8 Jin ZY, Teng Y, Wang HZ, Yang HL, Lu YJ, Gan MF. Comparative Analysis of Cage Subsidence in Anterior Cervical Decompression and Fusion: Zero Profile Anchored Spacer (ROI-C) vs. Conventional Cage and Plate Construct. Front Surg. 2021;8:736680. Published 2021 Oct 27. doi:10.3389/fsurg.2021.736680

